Strong Alkali Examples
In a reaction in water neutralization results in there being no excess of hydrogen or hydroxide ions present in the solution. The fine modern Gothic church of St John.
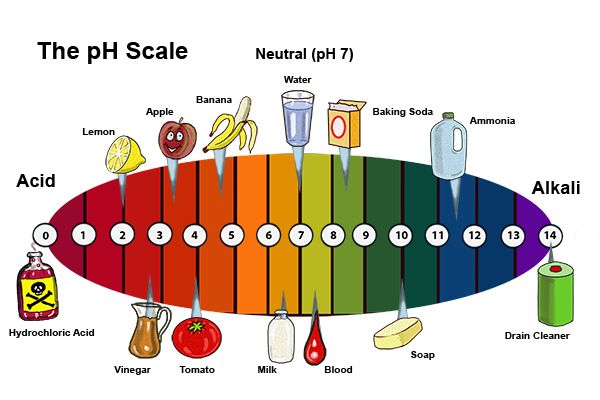
Bases Naming And Formulas Study Guide Inspirit
Alkali Metals.
. It is also known as lithium hydrate and lithium hydroxid. They are highly reactive elements distinctive because of their 1 oxidation state and generally low density compared to other metals. When alkali base reacts with metal it produces salt and.
Its primary use is for the synthesis of lubricating grease. Ashes of the saltwort is a basic ionic salt of an alkali metal or an alkaline earth metalAn alkali can also be defined as a base that dissolves in waterA solution of a soluble base has a pH greater than 70. Animal and vegetable fats and oils are made of ester molecules called triglycerides.
Lithium hydroxide is the weakest base of the alkali metal hydroxides. Sodium hydroxide magnesium hydroxide calcium hydroxide etc. Visit BYJUS to know the saponification process saponification reactions saponification value with Videos and FAQS in detail.
Alkali metals are in group IA on the far left side of the periodic table. The pH of the neutralized solution depends on the acid strength of the reactants. Note that the strong bases consist of a hydroxide ion OH- and an element from either the alkali or alkaline earth metals.
Soaps are widely used in bathing cleaning washing and in other household chores. The Leonhardskirche also a Gothic building of the 15th century. Biosystems can use this interaction through adjacent cationicaromatic amino acids sequence of.
The general equation of. The adjective alkaline and less often alkalescent is commonly used in English. Because they are so reactive these elements are found in compounds.
It is a white crystalline solid that readily reacts with water and is slightly soluble in ethanol. Of the numerous churches in the city the most interesting are the Stiftskirche with two towers a fine specimen of 15th-century Gothic. Fats have the specific gravity less than 1 and also lower than water therefore they float on water.
It is strong used in making steel. A substance used to repel something. LiOH Lithium hydroxide is a strong base.
Alkali is considered a strong base. Chemical properties of bases. An ester is a molecule that is formed from an alcohol and an acid.
Only hydrogen is found free in nature as a pure. The melting point of fats depends upon their constituent fatty acids. These are also known as alkali.
The hydroxide of alkali and alkaline earth metals are soluble in water. The Hospitalkirche restored in 1841 the cloisters of which contain the tomb of Johann Reuchlin. Fatty acids form salts with alkali and alkaline earth metal.
A chemical element that is a common greyish-coloured metal. This means when the strong acid is placed in a solution such as water all of the strong acid will dissociate into its ions as opposed to a weak acid. In chemistry an alkali ˈ æ l k ə l aɪ.
Salts of sodium potassium calcium and magnesium are formed when fatty acids react with these salts. In the case of fats glycerin is the alcohol and the acids are. Electrostatic interaction is strong but usually diminishes in high ionic-strength environments.
I Reaction of Base with Metals. An acid that is completely ionized in aqueous solution. Saponification is a type of chemical reaction between a strong alkali or base such as sodium or potassium hydroxide and a fat.
Saponification - Saponification is the hydrolysis of an ester with NaOH or KOH to give alcohol and sodium or potassium salt of the acid. The new Roman Catholic church of St. In chemistry neutralization or neutralisation see spelling differences is a chemical reaction in which acid and a base react quantitatively with each other.
Making you feel strong disapproval and that you do not.

What Is An Alkali Lesson For Kids Study Com
Examples In Everyday Life Acids Vs Bases

List Of Strong Bases 7 Examples Chemistry Teachoo

Acids And Bases Vocabulary Have Your Students Put Key Vocabulary Into Practice One Of The Things Students Can Find Really Di Teacher Guides Lesson Ph Chart
0 Response to "Strong Alkali Examples"
Post a Comment